Ethanol and Methanol
Click here to read the rest: hydrogen, Sustainable Aviation Fuel, ethanol, and biogas.
Ethanol and methanol always sounded confusing and boring to me. But they're actually pretty interesting.
This post will explain everything you need to know, from scratch.
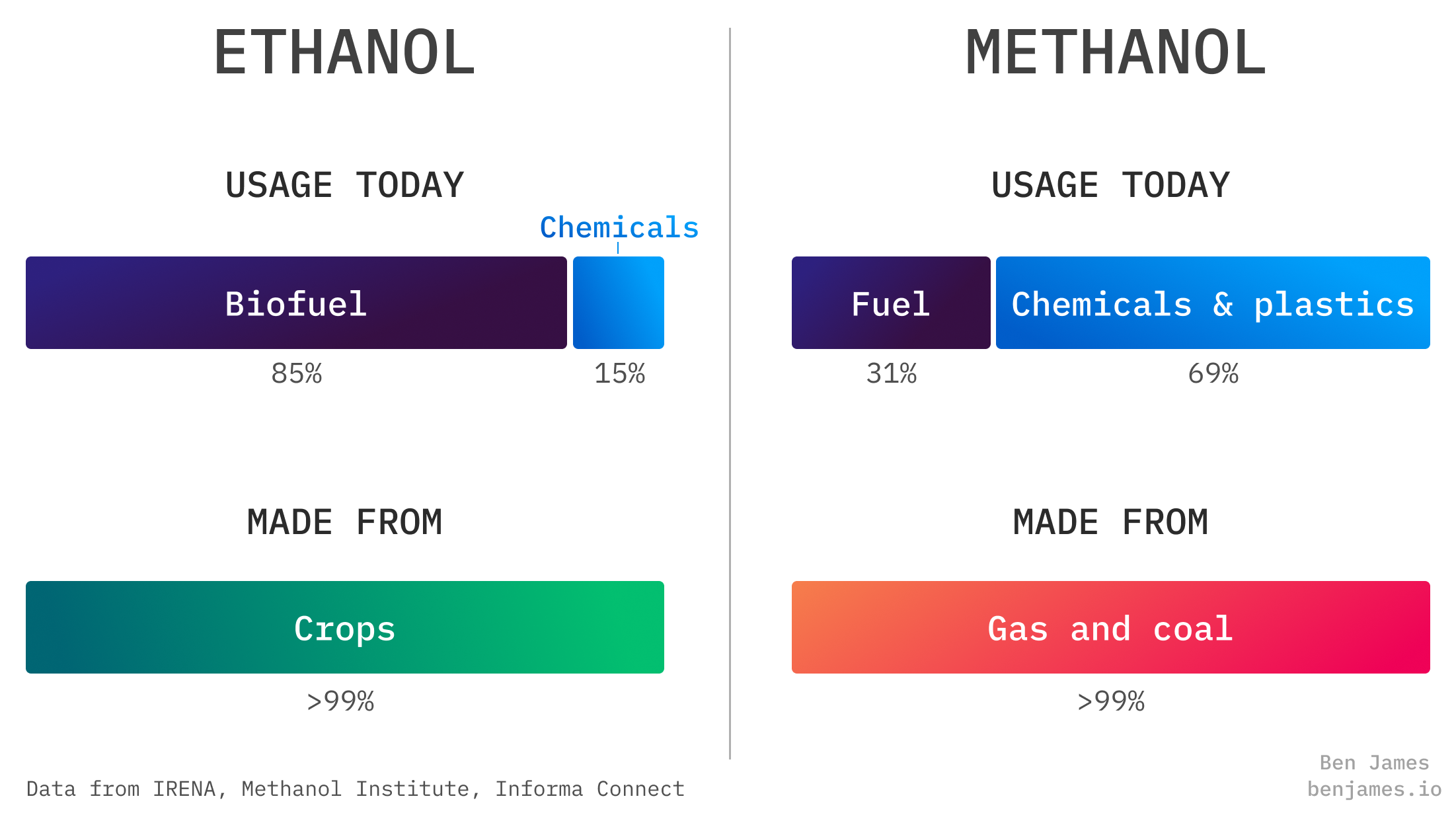
Ethanol and methanol are used for very different things.
We make ethanol and methanol in roughly equal quantities. As a rule of thumb: every year we make around:
- 100 million tonnes of hydrogen
- 100 million tonnes of ethanol
- 100 million tonnes of methanol
The basics
Most ethanol today is used as biofuel for cars. When people talk about ethanol for climate, they usually just mean making more biofuel. This would be used for cars (burned directly) or planes (as an ingredient in SAF).
Most methanol today is used for chemicals, though roughly a third is used in cars. When people talk about methanol for decarbonisation, they usually mean using it as a fuel. This would be for ships (used directly) or planes (as an ingredient in SAF).
Hol' up, these guys still make CO2 when u burn them?
Yeah. Ethanol and methanol both contain carbon, so they emit CO2 when burned. Ethanol produces slightly less CO2 than gasoline, and methanol produces slightly less CO2 than shipping fuel. But not by much.
So what makes them any better than fossil fuels? The difference is where the carbon comes from. If the carbon in your fuel comes from the air, not the ground, you’re not adding extra CO2 to the atmosphere.
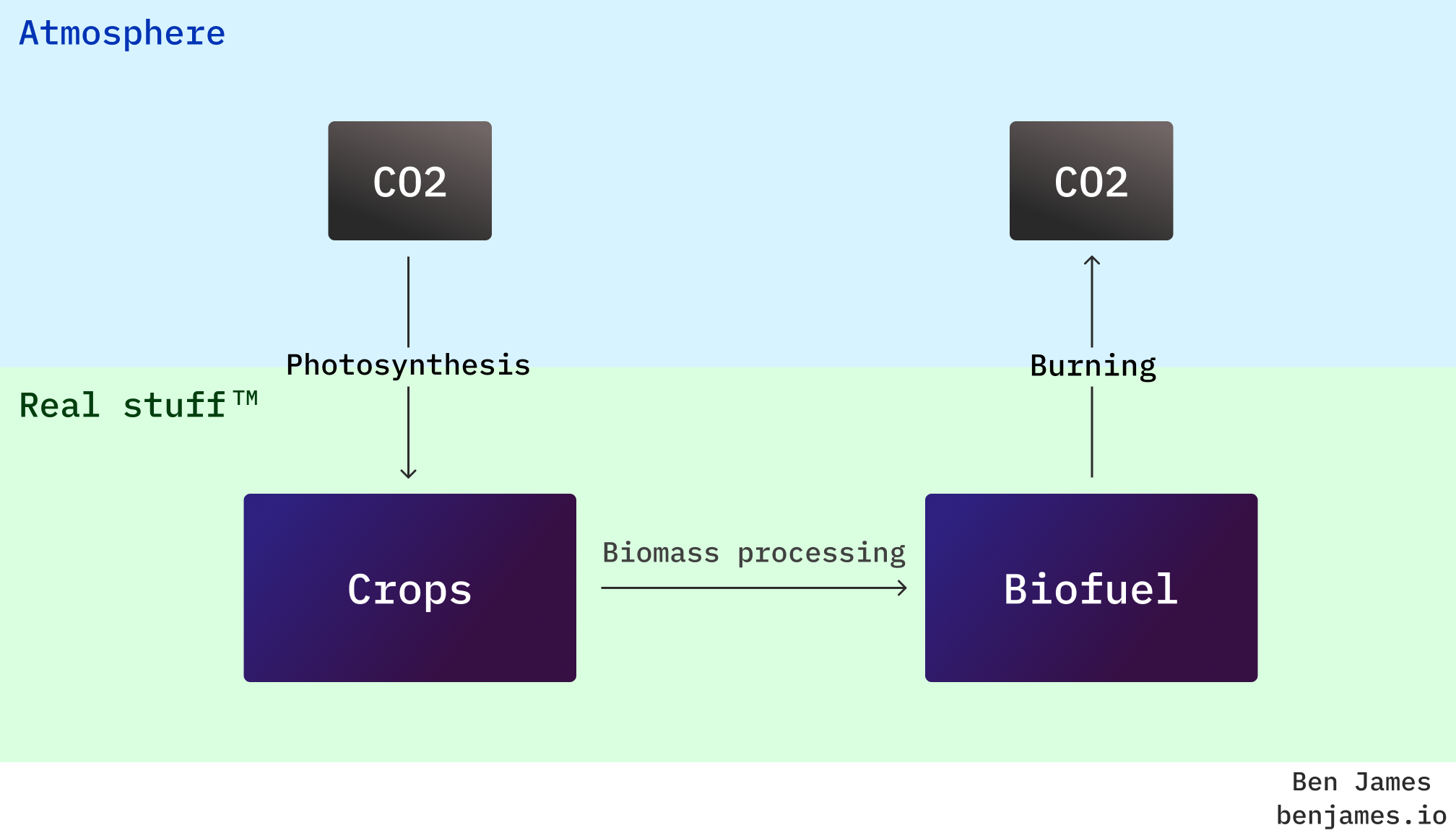
In theory, this means that fossil fuels add new CO2 to the atmosphere, whilst biofuels “recycle” what’s already there. In practise, the lifecycle emissions of biofuels are more dubious, but we’ll cover that below.
Ethanol for cars, methanol for ships.
Both methanol and ethanol have lower energy densities than fossil fuels, but methanol has the worst.
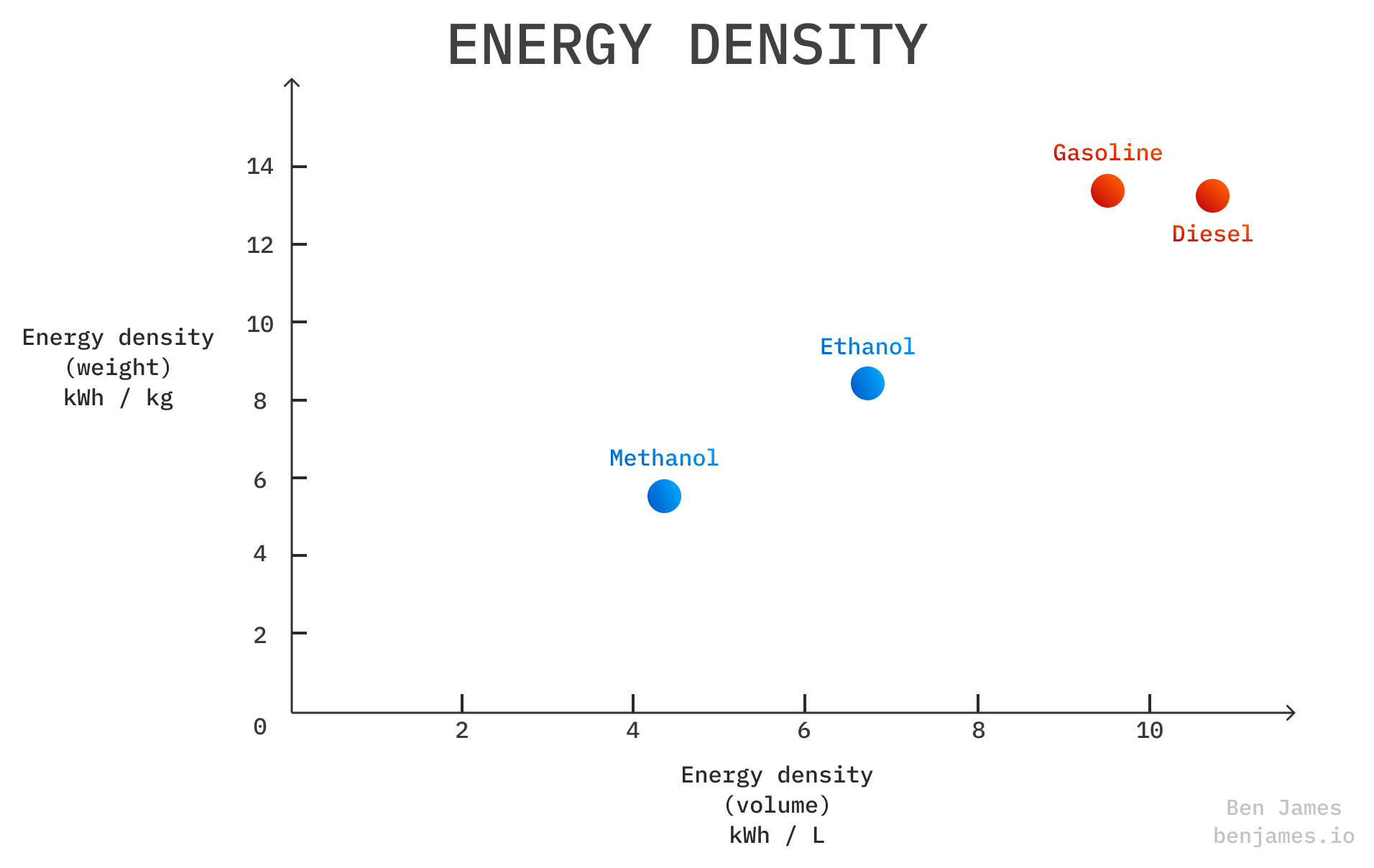
This is one natural reason why ethanol suits cars and methanol suits ships.
Additionally:
- Methanol is nastier. It’s a boring level of toxicity - nothing dramatic, but not friendly to humans.
- Methanol storage is already common in ports, because of its extensive use in the chemical industry
- Methanol has historically been a tad cheaper than ethanol, though not by much.
Ok, now let’s dive into them one by one.
Ethanol
Ethanol is the most widely used biofuel in the world. Production and usage are dominated by two countries:
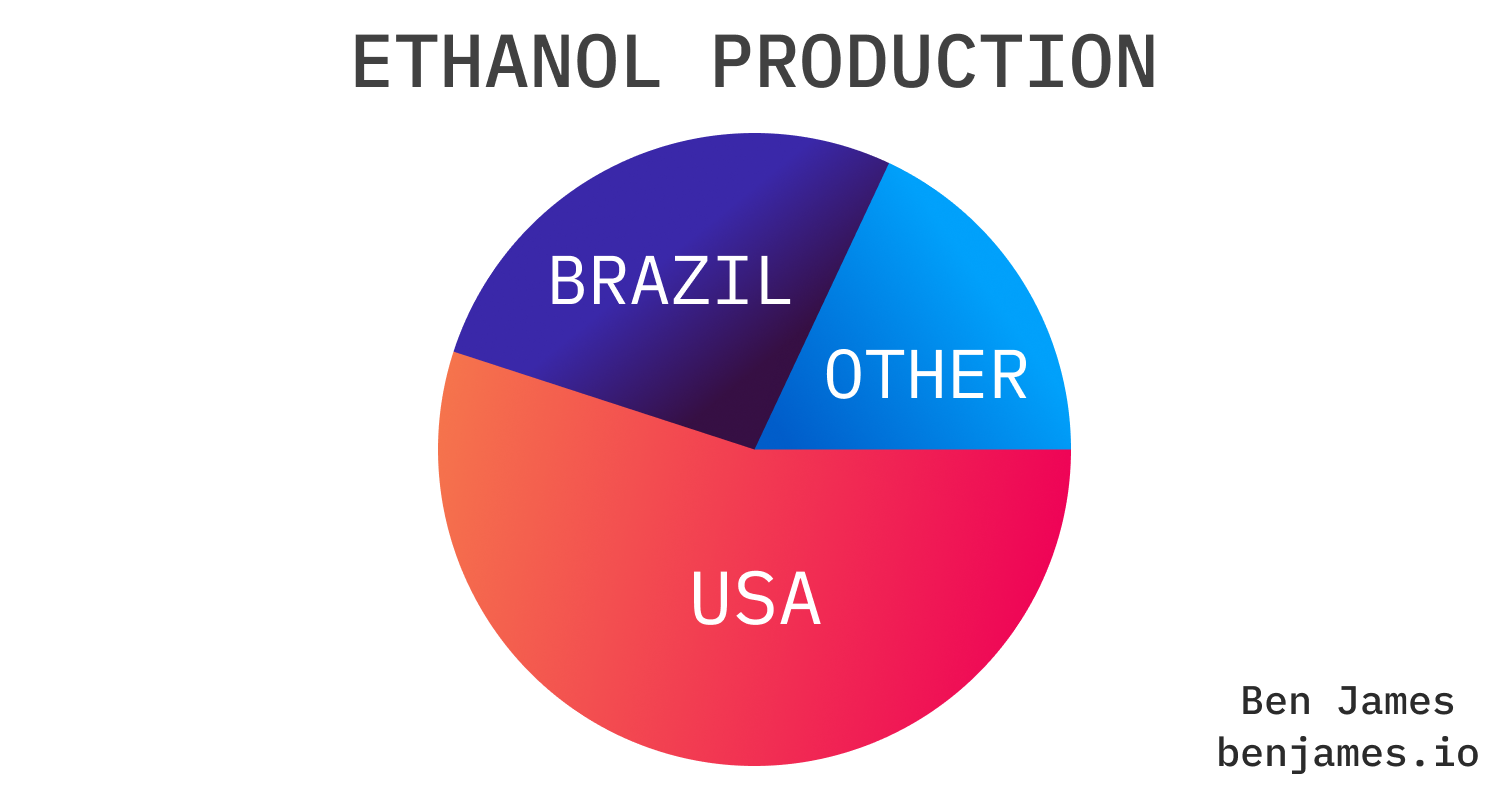
The US and Brazil are raging ethanol junkies. They use almost all for cars and motorbikes.
Brazil uses so much ethanol that no Brazilian cars run on pure gasoline. There is a minimum blend of 27% ethanol in all fuel, and 80% of light vehicles are special “Flexfuel” varieties, which can run on up to 100% ethanol.
Ethanol production
We make ethanol via fermentation. It’s literally the same thing as making beer. “Alcohol” (the drink, not the chemistry term), actually just means ethanol.
Fermentation is pretty simple. Just add sugar to some lukewarm, yeasty water.
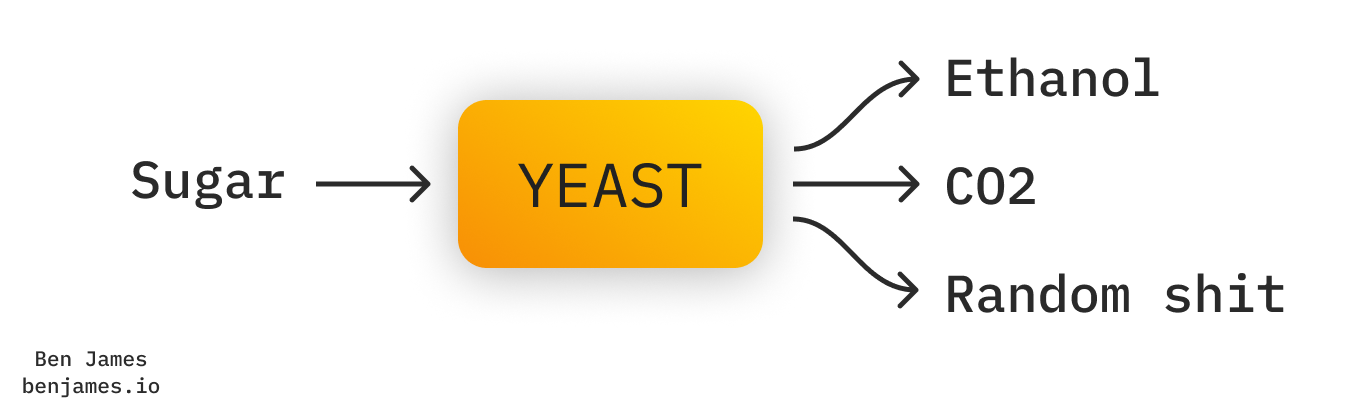
Producing ethanol also produces CO2. But notably, the CO2 that gets emitted is 100% pure. That’s great news, because it means that it’s easy to capture. By contrast, most industrial processes emit CO2 that’s much more highly diluted.
In fact, capturing CO2 from ethanol production is so easy that ethanol production is the biggest source of purified CO2 in the US, and the second biggest source in Europe. This is where we source the CO2 that we use in fizzy drinks, food production, fire extinguishers, etc.
Here’s an ethanol plant in Minnesota:

First-generation ethanol
The US makes its ethanol from corn, whilst Brazil makes its ethanol from sugarcane. Both are simple processes that are mature and well-established. Corn ethanol and sugarcane ethanol are known as first-generation biofuels.
Corn and sugar are easy to turn into ethanol, but they’re also… edible. That means that we’re using agricultural land that could otherwise be used for producing food for humans. It would be great if we could make ethanol without competing with food production…
Second-generation ethanol
An alternative source of ethanol is cellulose, the stringy and fibrous bits of a plant. You can get cellulose from grass, trees, and loads of different types of plants. It’s everywhere, but it’s harder to make ethanol from.
Second-generation biofuels mean biofuels made from non-food crops.
Cellulosic ethanol is the largest potential source of biofuel in the future, because about half of all plant biomass is cellulose. But whilst sugar and corn are easy to break down into ethanol, cellulose is a lot more complicated. It’s not yet clear that we’ll be able to do it cost effectively.
Unfortunately, corn ethanol stayed cheap, and cellulosic ethanol stayed expensive. Most plants shut down, and today no-one commercially produces cellulosic ethanol in the US.
What about ethanol from waste?
People love to talk about making ethanol from food and ag waste. That’s great, but we don’t do much of it at the moment. In the US, more than 95% of ethanol comes from virgin energy crops.
We should do more, but there's only so much food waste.
In our last piece on biogas, we discussed using food waste to make biogas. So should we use it to make biogas or ethanol?
A biogas plant is basically a big tank with a mixing spoon - it’s cheap. An ethanol plant is more complex, but makes a more versatile, easy-to-transport fuel. If you can afford it, making ethanol from waste allows you to do more epic stuff with your food waste, like fuelling planes.
Ethanol in planes
One exciting future use for ethanol is in jet fuel.
Scaling ethanol to replace gasoline is kinda silly, because we already have an easy way to decarbonise cars: EVs. By contrast, we don’t have an easy way to decarbonise planes.
To be clear, we won’t fuel planes with ethanol directly. Instead, we’ll use an Alcohol-to-Jet process that turns ethanol into synthetic kerosene - a drop-in replacement for fossil jet fuel. More on that in my next piece on SAF.
Ethanol has some haters.
There are two controversial things about ethanol. They mostly pertain to its use today.
Emissions reduction
Most ethanol today is used to displace gasoline, and the emissions savings from doing this are highly contentious. This is because there are plenty of GHG emissions in the life cycle of producing ethanol, for example: tractors, fertilisers, and uncaptured CO2 during fermentation.
Ethanol’s lifecycle emissions are famously hard to quantify, and some people even suggest that ethanol is worse than gasoline. Studies vary a lot, but most that I’ve found suggest US corn ethanol reduces emissions by roughly 40-50% versus gasoline.
Land use
Today, most ethanol production consumes crops that use vast areas of land. Land that could otherwise be used for food.
Corn is by far the biggest crop produced in the US, and a hefty chunk of it goes to ethanol production.

Ethanol production is also highly subsidised in the US. From an energy lens, the US ethanol programme is bloated and unfit for purpose. It sticks around because it is primarily a political decision to subsidise US farmers.
Alright! Time to switch gears and dive into methanol.
Methanol
Today, most methanol is used in the chemicals industry. Methanol is one of the four basic building blocks that is used to make most other complex chemicals (the other ones are ethylene, propylene and ammonia).
Most talk of methanol for decarbonisation focuses on methanol as a fuel - usually for shipping and aviation.
Methanol for shipping
Methanol is considered one of the main contenders to decarbonise shipping. Other approaches include ammonia, carbon-capture-on-ships, and (surprisingly) big, heavy batteries.
Methanol’s primary advantage is that it doesn’t require huge ship modifications. Because it’s already an internationally shipped commodity, ports have existing methanol storage (over 100 major ports), and are used to handling it.

That doesn’t mean that methanol for shipping is all roses. We’ll cover the energy requirements below.
Can methanol do planes too?
You betcha - methanol-to-jet is a hot topic. BUT methanol is specifically excluded from the list of approved SAF production pathways. That means it’s nearly impossible to sell commercially atm.
More on that in my next piece on SAF.
Methanol production
Methanol’s chemical symbol is CH3OH. The important part is that it contains a “C” for carbon. Ie, to make methanol, we need a source of carbon.
Today, that carbon comes from fossil fuels. In China, it’s coal; in the rest of the world, natural gas. Before we think about making more methanol, we first need to decarbonise the production of our existing methanol.
There are two ways to make clean methanol - and both have different sources of carbon:
- Biomethanol uses carbon from biomass (eg plants or household waste).
- E-methanol uses carbon from CO2 captured from industrial processes, or pulled straight from the atmosphere (Direct Air Capture).
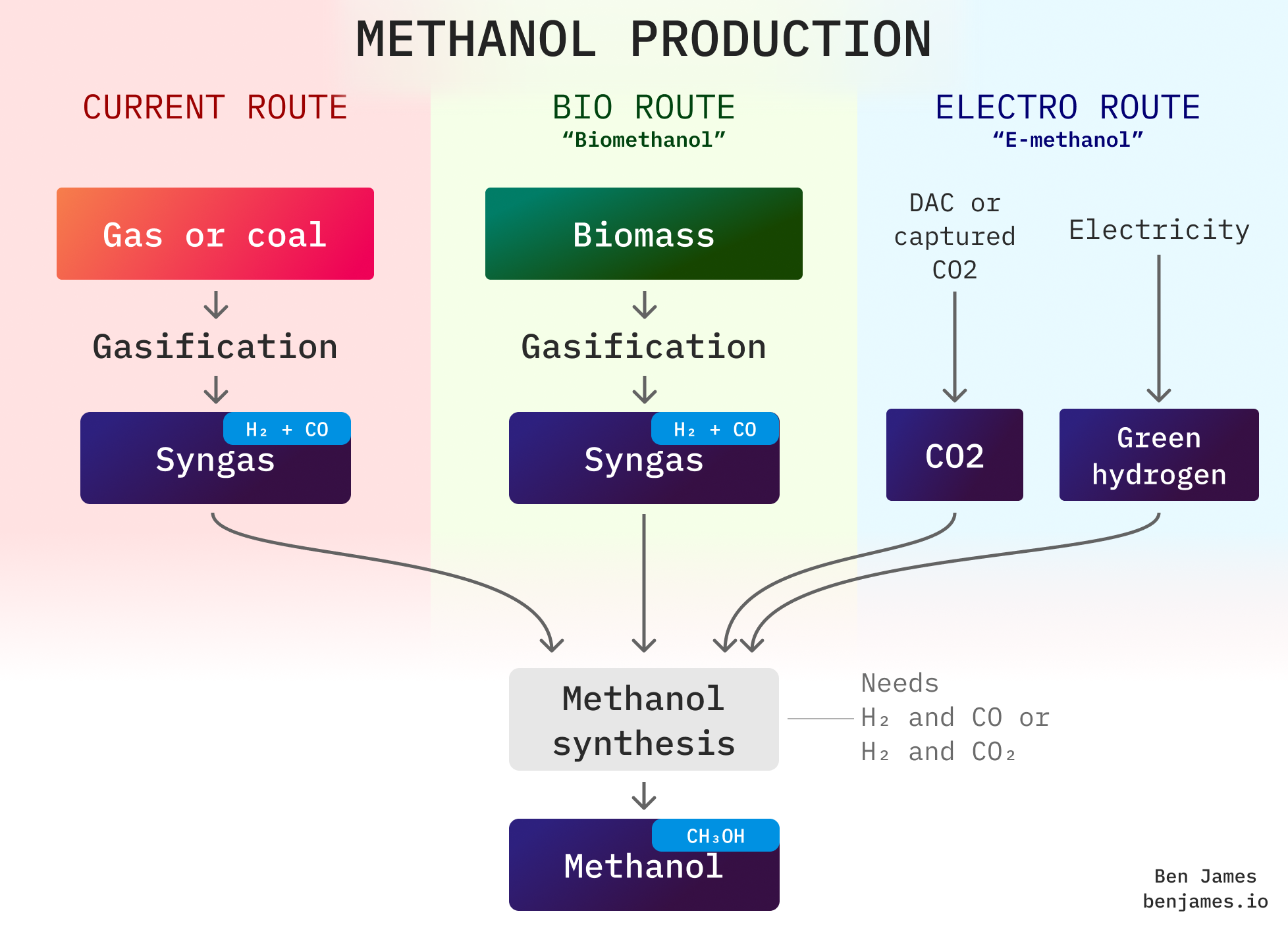
The fossil fuel route and bio route both use something called “syngas”. What’s that?
Syngas is made by a process called gasification, which is performed on things that contain carbon, like biomass or fossil fuels. Gasification just means hotting something up real good (at least 700 °C) whilst limiting the oxygen supply. It’s a bit like partially burning something.
It produces a mix of carbon monoxide (CO) and hydrogen (H2), which we call syngas.
Fun fact: up until the 60s/70s, the US and UK piped syngas to peoples’ homes for heating. It was only after the 60s that we switched to piping natural gas instead.
Sourcing carbon for methanol
The bio route sources carbon from biomass that originally absorbed CO2 from the atmosphere. This includes:
- Agricultural waste (twigs, stalks, grass, etc)
- Leftovers from making pulp and paper
- Municipal waste
- Sewage
- Biogas (which I explain here), though this requires reformation, not gasification to make syngas.
The electro route sources carbon from CO2 streams. This includes:
- CO2 captured from industrial plants using fossil fuels. This is bad, because the CO2 didn’t come from the atmosphere originally.
- CO2 captured from industrial plants using biomass. For example, plants that make ethanol! This is better, because the CO2 came from the atmosphere originally - however, sourcing non-bullshit sustainable biomass is tricky.
- CO2 removed directly from the atmosphere, using Direct Air Capture. This is the best source of CO2, since it unequivocally came from the atmosphere originally. However, DAC has its own problems!
E-methanol is a bit needy 😇
Let’s revisit our production diagram:
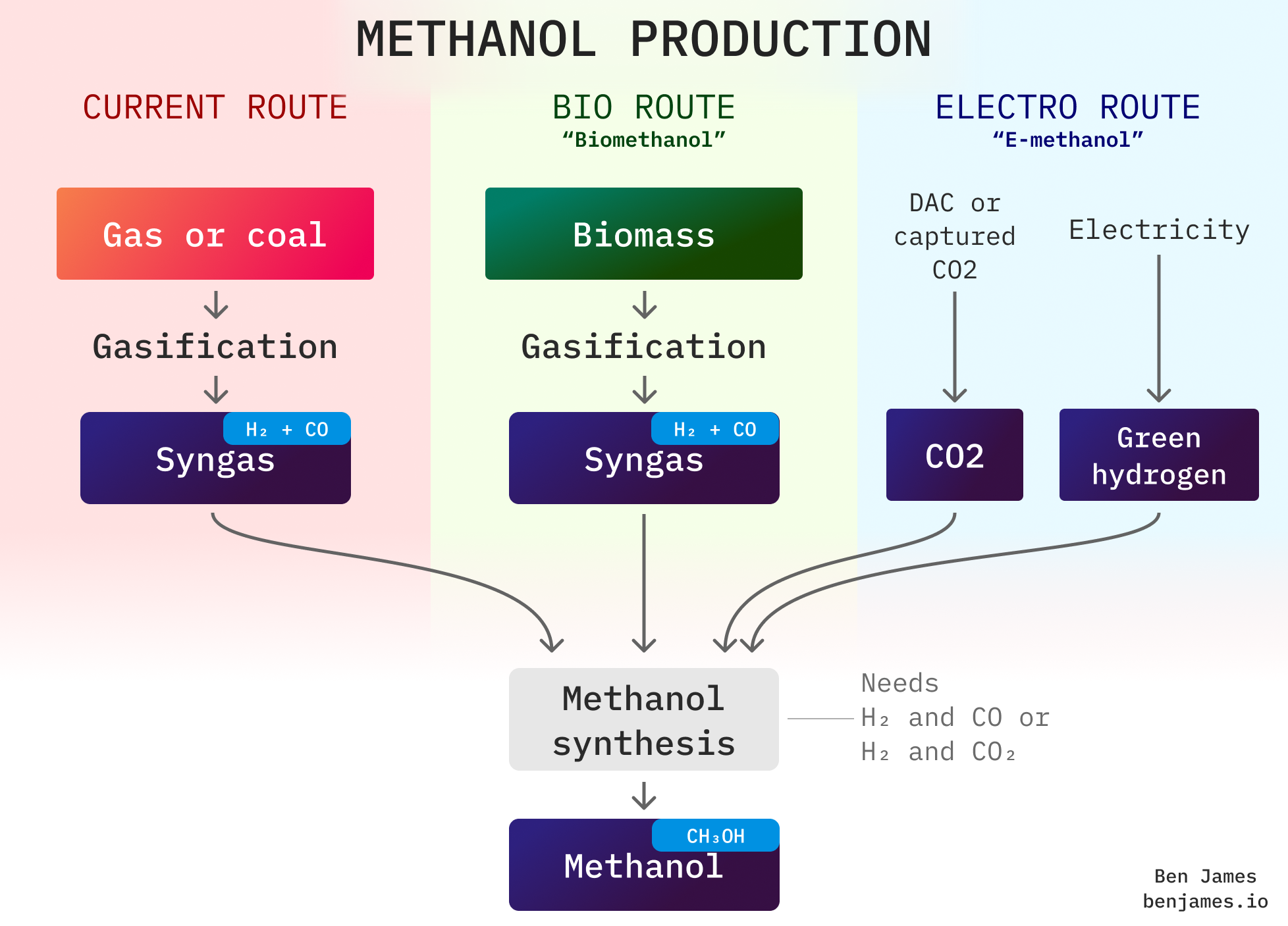
Going the electro route requires the critical ingredient of green hydrogen. And remember, green hydrogen needs a lot of electricity.
As a rule of thumb, you need about 10 MWh of electricity to produce 1 ton of e-methanol. Almost all of this is for hydrogen, and this doesn’t include energy for powering DAC.
To replace just our current methanol production (100 Mt / yr) with e-methanol, we would need around 1000 TWh of electricity. That’s roughly the electricity consumption of the UK, Germany, and the Netherlands combined. Crucially, that’s before we even start making any more methanol to use as a fuel.
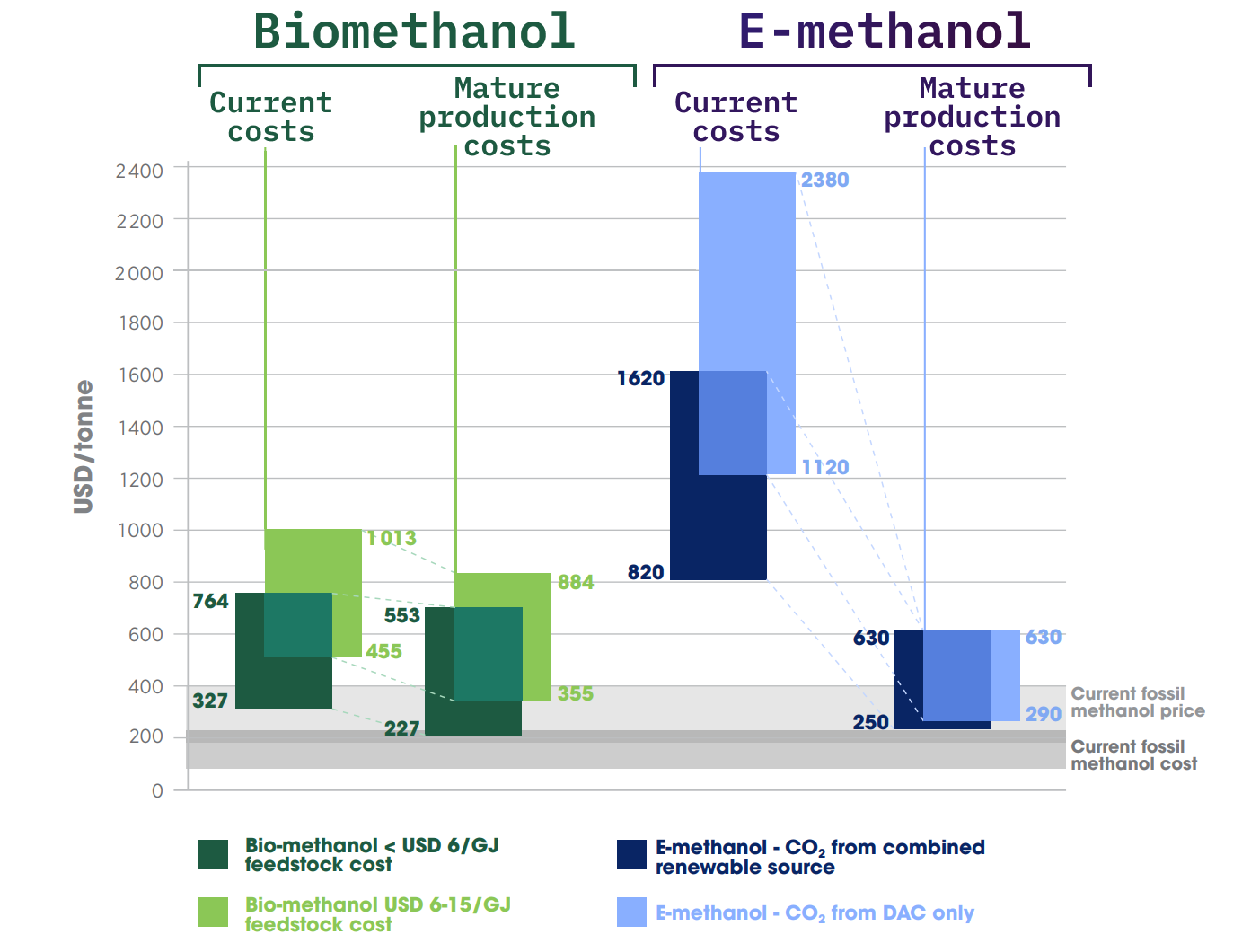
This means e-methanol is pretty costly, especially when you factor in the costs of DAC.
Conclusion
That’s all for ethanol and methanol!
Here’s a cheat sheet of the important questions to ask about production projects.

Click here to read the rest on hydrogen, Sustainable Aviation Fuel, ethanol, and more.