Ammonia - the basics
Click here to read the rest on hydrogen, Sustainable Aviation Fuel, ethanol, and more.
I’ll start with the spoilers.
- Ammonia is the biggest CO2 emitter in the chemical industry.
- Ammonia makes a terrible fuel. It’s just a more expensive form of hydrogen. And it’s toxic.
- Using ammonia for electricity generation is bullshit, in Europe and the US.
- But ammonia is easier to transport by ship than hydrogen. Because of this, Japan, Korea, and other energy-poor countries plan to import ammonia.
Let’s begin with the basics.
30 second ammonia crash course
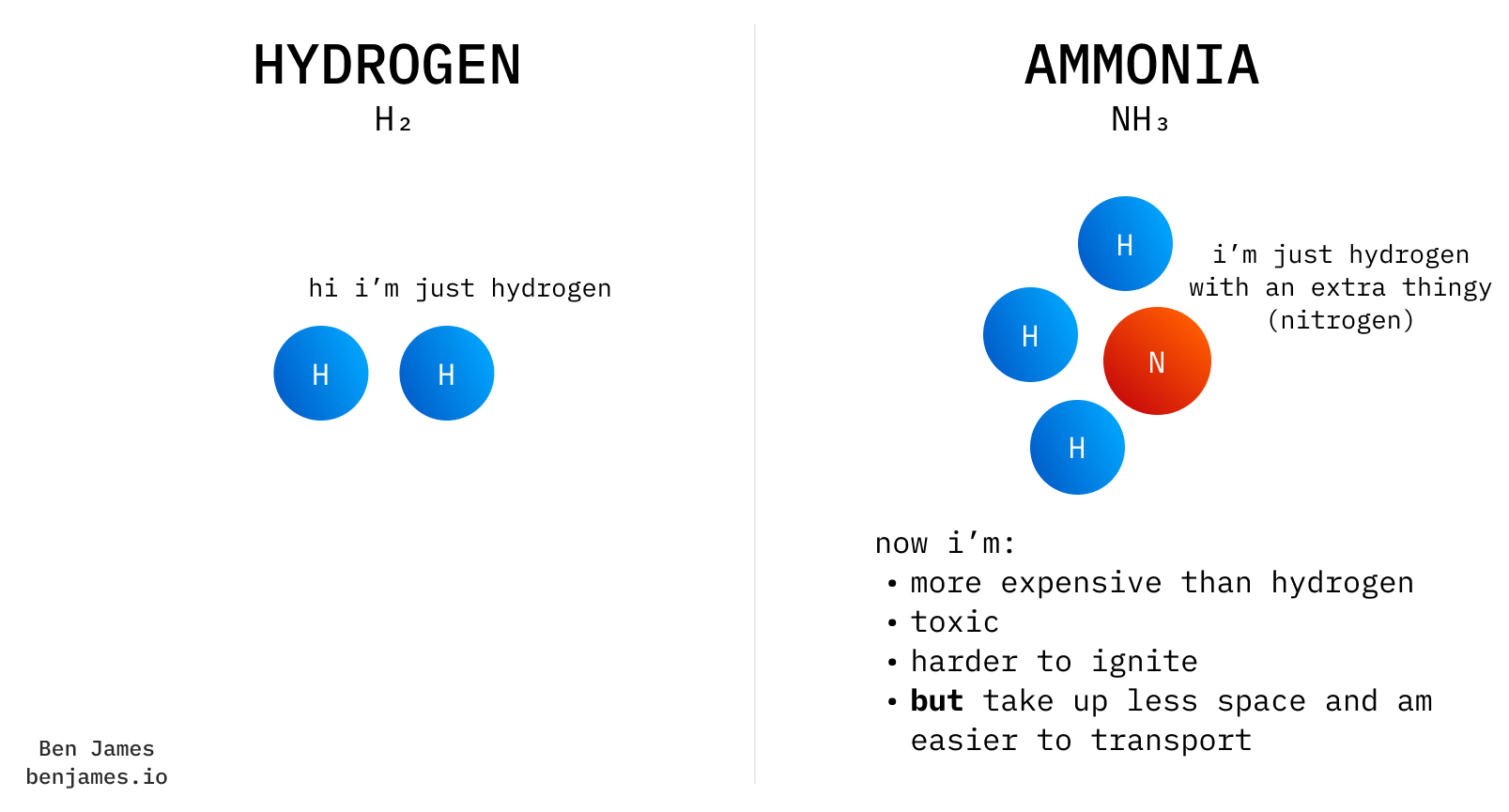
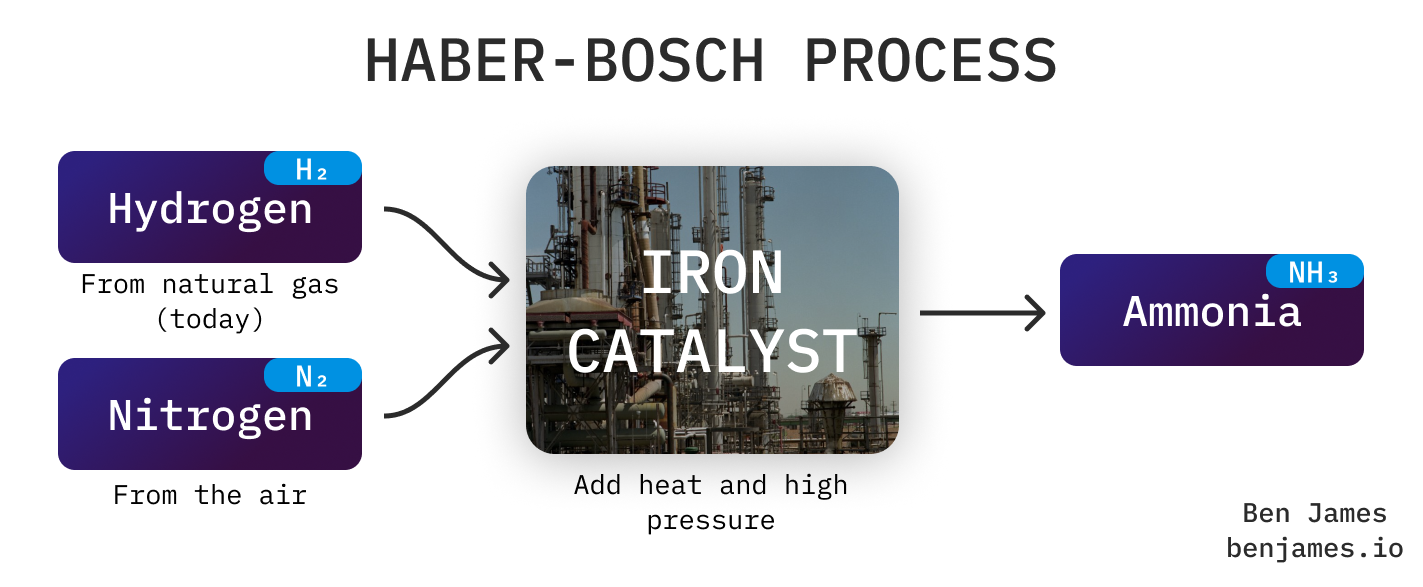
Ammonia is made of hydrogen and nitrogen.
Today, we use ammonia for its nitrogen (we make nitrogen fertiliser).
In the future, we might use ammonia for its hydrogen (for energy).
In the context of energy, ammonia is just fancy hydrogen.
In 2024 almost all ammonia is used for making fertiliser. Without ammonia, 8 billion people could not be alive at once. But producing it is dirty - ammonia is 45% of emissions from the chemical sector.
In the future, we’ll clean up our existing ammonia production. We might also use it for new things.
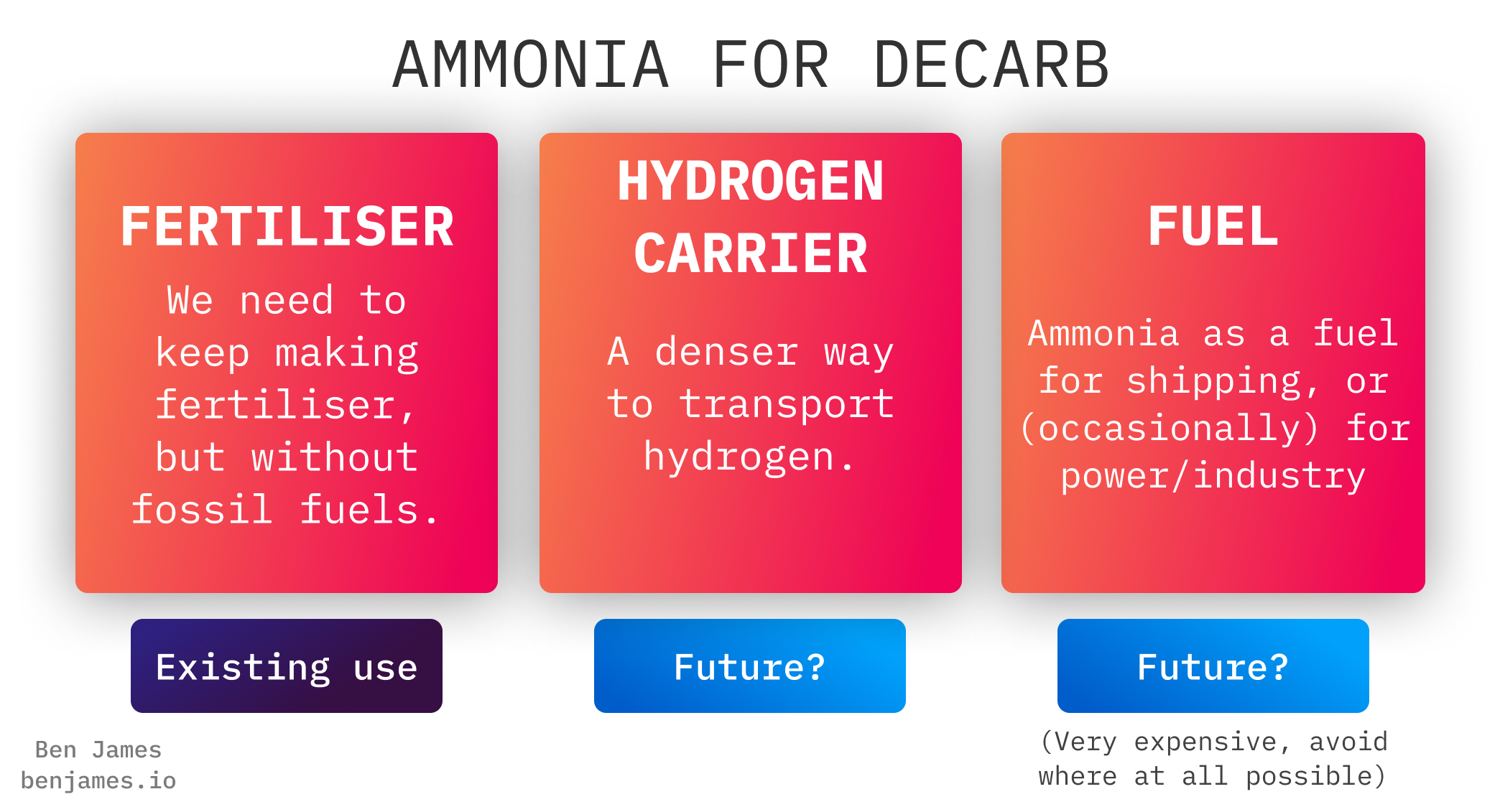
This blog will walk through these three categories.
#1 - Fertiliser
Plants grow better when you add nitrogen.
In the 1800s, Europeans’ eyes were popping out their sockets at how fast their crops were growing after they added guano (bird poop, which contains nitrogen). They found some islands near Peru with loads of guano and cheerfully mined the heck out of them.
But by the early 1900s, the guano was running out. Two German chemists invented the Haber-Bosch process - the first decent industrial process for producing ammonia. Ammonia contains nitrogen, so this meant that for the first time ever, humanity could produce nitrogen fertiliser on demand.
The Haber-Bosch process is probably humanity’s most important invention, because it feeds most of the global population today. Roughly half of the nitrogen inside your body came from the Haber-Bosch process.
Unfortunately Haber-Bosch plants today are mostly fed by natural gas, which is a total bummer.
Ammonia production today
Here’s how we make fertiliser today.
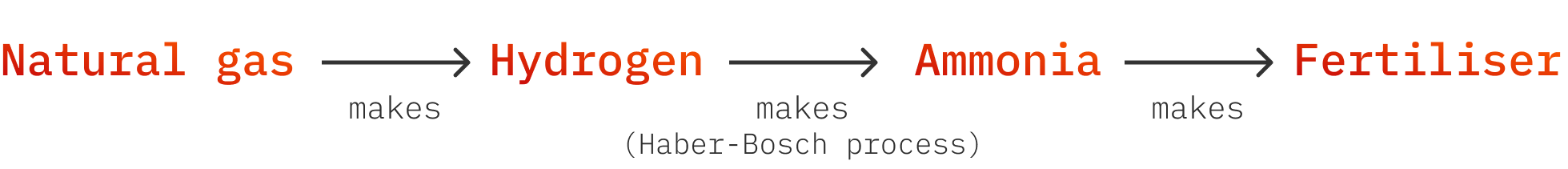
Let’s zoom in on the Haber-Bosch process.
We make three types of nitrogen fertiliser from ammonia: nitrates, urea, and just raw ammonia. They each have different CO2 and NOx emissions in production and on the field.
But all types of nitrogen fertiliser emit a lot of CO2 in production, because they use dirty hydrogen made from fossil fuels.
We need CO2 for fizzy drinks, food production, fire extinguishers, etc. In Europe, we get most of this CO2 from ammonia production. We capture it from the grey hydrogen produced on-site.
This means that the CO2 supply chain is reliant on the fertiliser sector. When gas prices soared in 2021, we made less fertiliser, and so there was a CO2 supply crisis. When ammonia production cleans up, we’ll have to find a different source of CO2.
(Unlike Europe, the US gets most of their CO2 from bio-ethanol production, which we’ll cover in part 4!)
Clean ammonia
So how do we clean up the process shown above?
There are two sources of emissions here.
- CO2 released from grey hydrogen production (see my guide to hydrogen for more on this)
- CO2 released from burning natural gas to heat up our Haber-Bosch plant.
Emissions from hydrogen (#1) are the most important. They make up between 75% and 90% of the CO2 emitted in ammonia production. That means you can clean up most of ammonia’s emissions by simply substituting grey hydrogen for green hydrogen. Right now, green hydrogen is much more expensive than grey hydrogen - but that might change soon.
Decarbonising #2 is hard. The race for the best solution is being run as we speak. The contenders are:
- Keep doing Haber-Bosch, but heat it with electricity or hydrogen. Haber-Bosch runs around 450°C, so replacing natural gas with electric or hydrogen heating is pricey (24/7 electricity typically costs around 3x more than gas). Note: the plant heats itself once started. Currently cold starts are rare, but intermittently-made electricity or hydrogen might change this in future.
- Electrochemical nitrogen fixation. This replaces the Haber-Bosch process entirely. Using two plates and some electricity, we can turn nitrogen and hydrogen into ammonia. I’m excited about this - particularly with AI-powered materials/catalyst discovery - but the electricity requirements are still eye-watering. Also, most current electrochemical processes for this require a lot of lithium.
- Bacteria. Again, this replaces Haber-Bosch. We can use bacteria to turn nitrogen into ammonia, but doing it at scale is tricky.
Cleaning up ammonia production is important, not just because of emissions. It also means that fertiliser and the price of food are more insulated from fossil fuel price spikes and the accompanying geopolitics.
Ammonia infrastructure
Because we already make fertiliser, some places already have infrastructure and laws for handling ammonia.
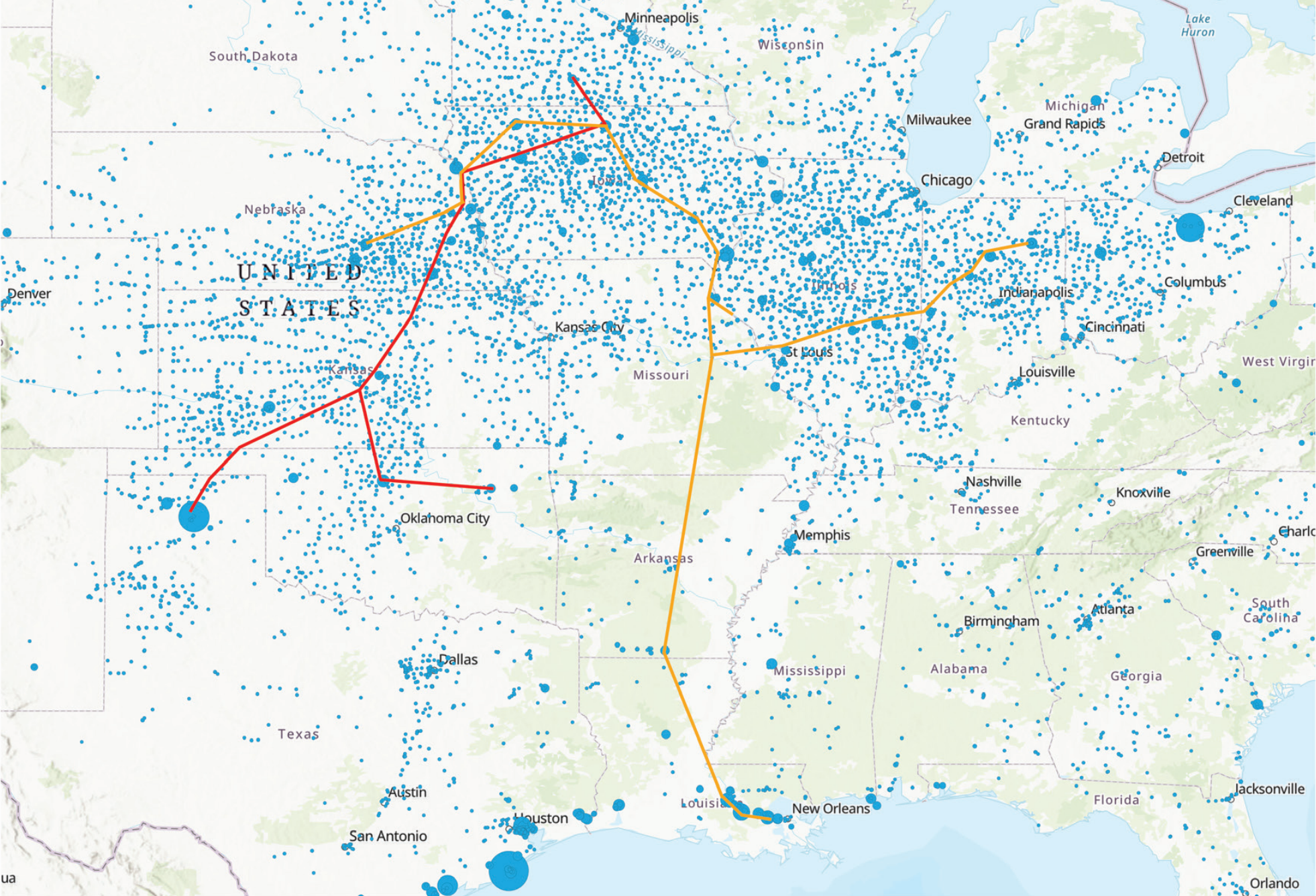
In the UK and Europe, we primarily use nitrate fertilisers. Nitrate fertilisers are made from ammonia, but shipped to farms as coarse powders. In the US, ammonia is applied directly to the soil. It’s stored as a pressurised liquid in tanks on the farm, and there are ammonia pipelines to transport it from gas-rich Texas and Louisiana up to the corn belt.
#2 - Ammonia as a hydrogen carrier
Here’s something that blows my mind: liquid ammonia contains 50% more hydrogen by volume than liquid hydrogen. You can transport the same amount of hydrogen, using less space, if you transport it as ammonia. Pure hydrogen is of course much lighter, but ammonia takes up less space.
Ammonia also turns into a liquid much easier than hydrogen. To turn hydrogen into a liquid, you have to squish it to 300x atmospheric pressure, or cool it to -253°C. For ammonia, it’s 10x atmospheric pressure, or -33°C.
That means that ammonia could be a convenient way to transport hydrogen over long distances. The idea goes like this:
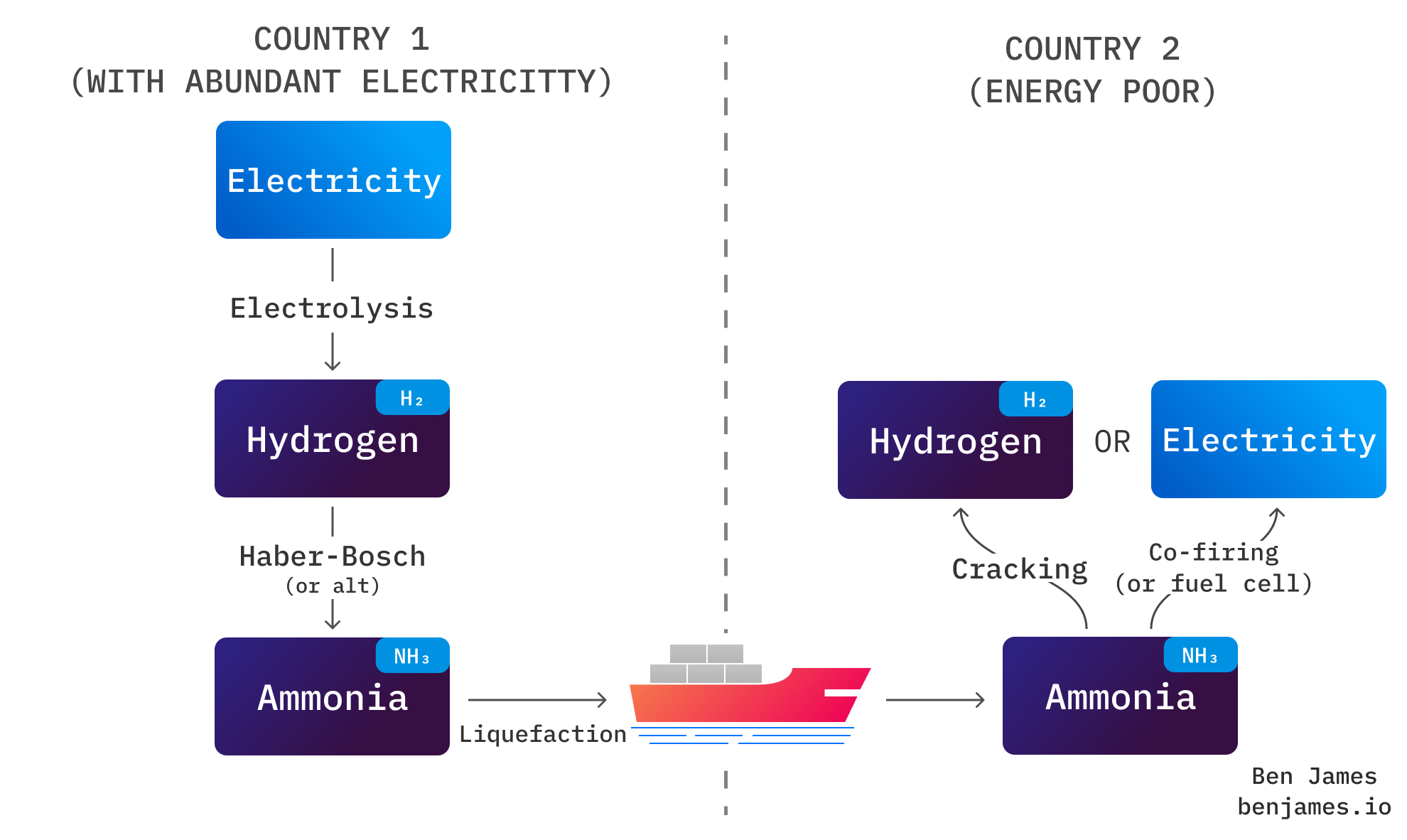
Upon reaching its destination, ammonia can be “cracked” back into hydrogen, or used directly as a fuel (which we’ll cover in section 3). Cracking just means splitting ammonia back into hydrogen and nitrogen. You heat it up to about 800°C over a nickel catalyst, and it pops right apart. Note: cracking takes a lot of energy!
This whole scheme sounds pretty neat on paper, but the real world is harsh.
The problem: ammonia is just more-expensive-hydrogen
Every conversion step in the picture above loses energy. You can expect to waste at least 70% of your energy - meaning that your electricity-to-electricity efficiency is 30% on a good day, and probably much lower.
It simply costs money to do this. Just converting hydrogen to ammonia adds roughly an extra $1 per kg of hydrogen. Remember from part 1 that we’re competing with grey hydrogen, which you can pick up for $1.50 per kg in the US.
So let’s be clear - it makes no sense to ship ammonia to places that can make their own hydrogen. No one sensible is proposing it.
However, for countries without the resources to produce hydrogen, importing ammonia could be useful. A good example is Japan.
Japan and ammonia
Japan drew the geographical short straw for energy production. It has a terrain of rugged mountains and forests, so solar capacity is limited. It has a deep seabed close to the coast, so conventional offshore wind is tricky too. After Fukushima, there is an understandable resistance to nuclear. Interconnecting with China is politically risky. And limited oil & gas fields mean that there is limited capacity for sequestering captured carbon.
Japan currently imports a lot of LNG (Liquefied Natural Gas) on big ships. They can’t use dirty natural gas forever - but they will still need to import some energy. Thus, they are placing bets that they will be able to import hydrogen and ammonia in the future. They've made some strong policy decisions to this effect. Korea and other smaller Asian countries aren't far behind.
Ammonia is shipped
It’s worth noting that ammonia is already shipped around a lot. Port infrastructure is already mature because of Big Fertiliser.
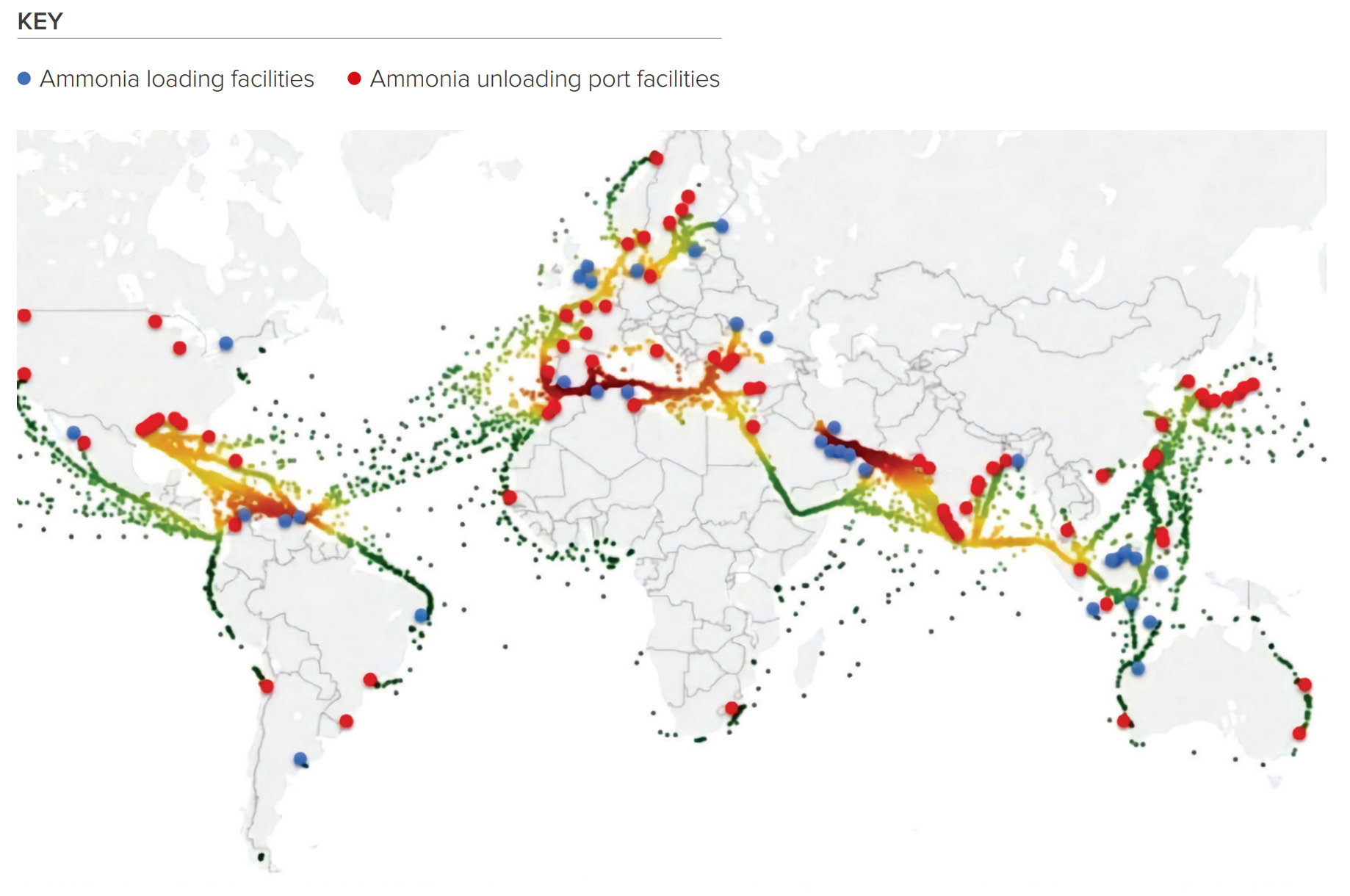
Not only does infrastructure already exist, but regulations do too. They’re important when you’re shipping a nasty chemical like ammonia.
It’s useful that the cost of ammonia transport is relatively small, because the spreads between clean ammonia prices in different countries will be big. Here’s the example of Singapore producing vs importing ammonia.
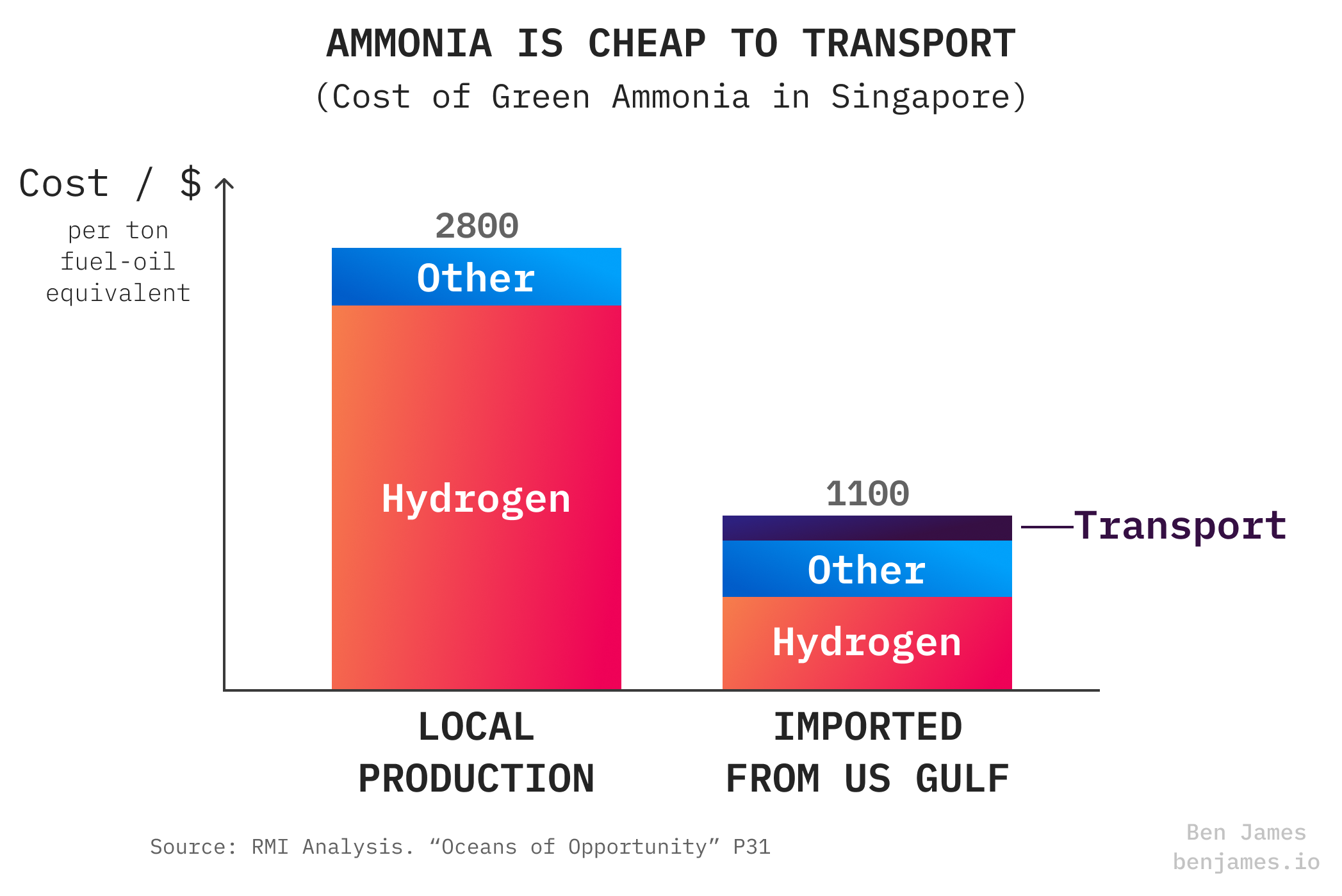
We know for a fact that green hydrogen production will cost wildly different amounts in different parts of the world. That’s because green hydrogen is dependent on renewable electricity, so places with lots of sun and wind will have a big price advantage.
Yes, shipping hydrogen via ammonia will waste a lot of energy. But if the green hydrogen is sufficiently cheaper at the source than the destination, that might not matter.
#3 - Fuel
Finally, let’s talk about using ammonia itself as a fuel. The two most-discussed applications are shipping and electricity production.
Why not cars, road vehicles and boilers/furnaces too? For starters, ammonia is even more inefficient than hydrogen. You will waste a lot of electricity making green hydrogen to make clean ammonia. You’re much better off using electricity directly, in an electric vehicle or heat pump.
Ammonia vs hydrogen
It’s worth emphasising this one:
And because hydrogen is made from natural gas or electricity, ammonia is inherently more expensive than the gas or electricity that was used to produce it.
In Europe and the US, no-one talks about ammonia as a fuel very much. That’s because we can make green hydrogen. If you have access to hydrogen as a fuel, you will use it directly instead of making ammonia. It’s only in places with limited hydrogen production (like Japan or Korea) where ammonia as a fuel is interesting.
Also, ammonia hates you.
Ammonia and human bodies are not pals. Ammonia burns and destroys human tissue, including eyes, lungs and probably most of your top 10 fave limbs. Inhaling ammonia can make you blind, or dead.
When ammonia is handled by industry, it’s centralised and contained, so it’s easier to keep safe. But using ammonia as a consumer fuel would be silly. In the US, where ammonia is used on farms, severe accidents are frequent.
Boom-boom vs zippity-zappity
There are two ways that you can use ammonia as a fuel. You can burn it, or put it through a fuel cell - just like hydrogen.
A fuel cell takes in ammonia, and outputs electricity. You can get 40-65% efficiency from an ammonia fuel cell, depending on how much you spend.
Meanwhile, burning ammonia produces heat, and as always, turning heat into useful energy is inefficient. You might get 35% efficiency in an internal combustion engine (heat to motion), or 55% in a combined cycle power plant (heat to electricity).
Ammonia is hard to ignite, and it has a low flame speed. That’s why people usually talk about co-firing ammonia: burning it with another fuel that can ignite and sustain the combustion (usually a fossil fuel).
Studying combustion is quite a big field in thermodynamics, but knowledge on ammonia combustion is pretty immature. Learning more about the nitty gritty of how best to burn ammonia is an active research area. Japan is a world leader.
One more thing - if you don’t combust ammonia completely (which is quite easy to do), the nitrogen in ammonia can produce nitrous oxide (NOx). This is extremely cringe and a massive self-own as NOx is a potent greenhouse gas (about ~300x the warming potential of CO2).
Ammonia for shipping.
Ammonia and methanol are the two big contenders for alternative maritime fuels.
We’ll have biodiesel, batteries, and maybe even carbon capture on some ships, but there remains a need for a renewable, clean, long-distance fuel.

Methanol is a hydrocarbon. That means it’s more similar to current fuels, so ships don’t need heavy modification. That’s an advantage, but also its achilles heel. The carbon that it contains has to be sourced from somewhere, and it’s expensive. We’ll cover methanol in depth in part 4 of this series!
Big ship-makers like Maersk are informally adopting the strategy of “methanol today, ammonia tomorrow”.
Ammonia for power generation
It’s possible to burn ammonia in a modified gas turbine or coal plant to produce electricity.
By now you should know where this is going: ammonia costs more than green hydrogen, and green hydrogen costs more than gas. Because ammonia is hard to ignite, it would likely be co-fired with fossil fuels.
Whilst it might be a necessary option for somewhere like Japan, that doesn’t mean it’s any cheaper.
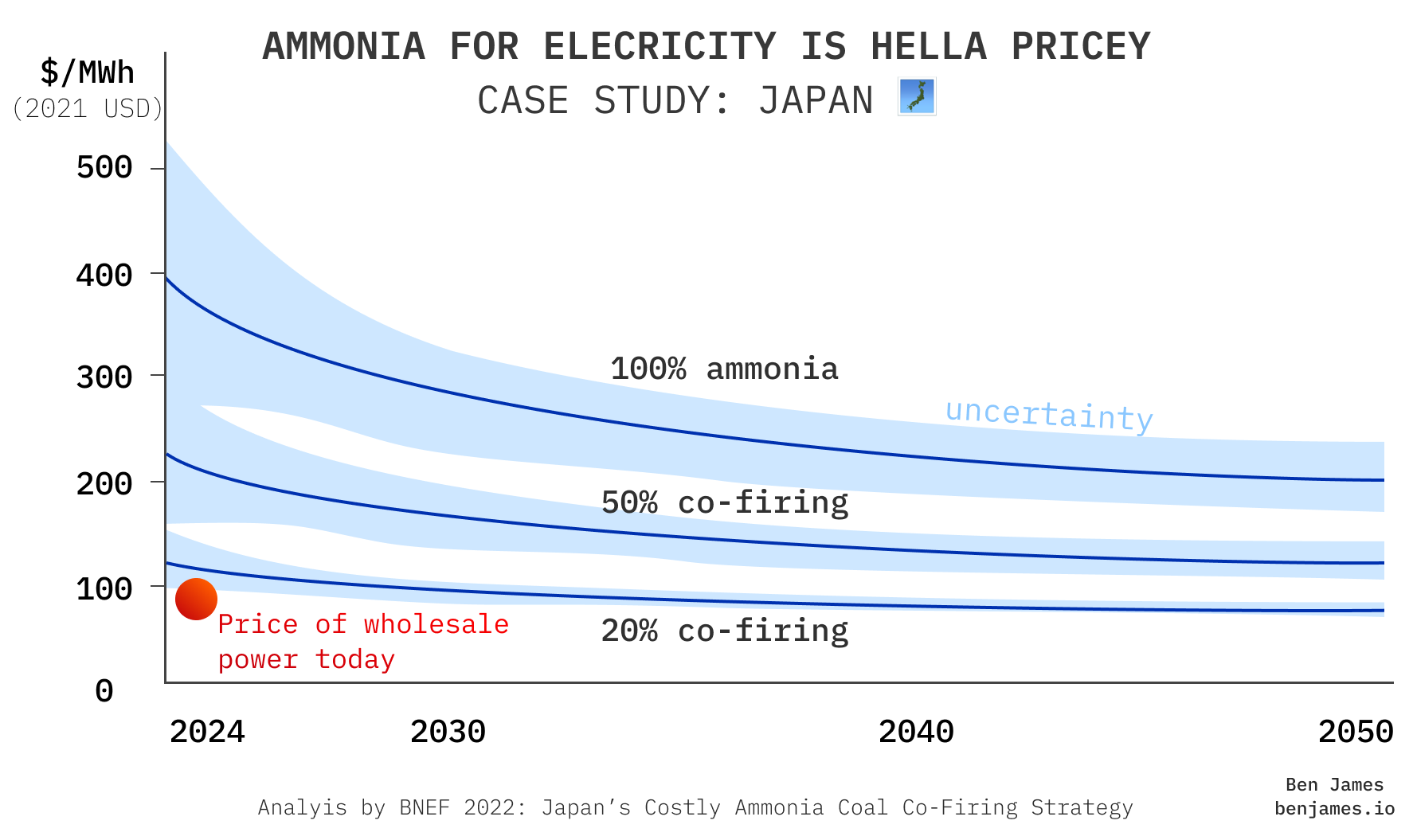
To be honest, I haven’t quite decided what I think about Japan's strategy here, because two extremely strong forces are colliding.
#1 - You can’t operate an industrial economy with expensive energy
Japan has a large manufacturing and industrial base that relies on cheap energy to be a competitive exporter.
Importing energy in the form of hydrogen, via ammonia, means that Japan’s energy costs will be far higher than most other countries. Ammonia is simply far more expensive than fossil fuels - at least for the medium term.
Switching to ammonia anytime soon would be like a self-imposed russian gas crisis. It would put a huge drag on energy prices, industry, and living costs - and make it difficult to be a competitive global exporter.
#2 - Cheap solar
Solar is getting stupidly cheap, year after year. This is unquestionably the most important fact for future energy.
If you take the PV price curve seriously and extrapolate it a decade or two, then we will have cheap green hydrogen. It will become cost-effective to build crappy electrolysers that can run on limited capacity factor solar, because energy is nearly free.
Since green hydrogen is the main cost centre for clean ammonia, cheap solar means cheap clean ammonia.
Summary
Ammonia is hard.
- It’s even more expensive than green hydrogen.
- It’s even less efficient than green hydrogen.
- It’s toxic, and hard to burn.
But it has some useful features.
- It’s more compact to transport than hydrogen.
- Unlike methanol, it doesn’t contain carbon. It doesn’t emit carbon when burned, and it doesn’t need to be made with expensive carbon from the atmosphere.
Huge thanks to Teja, Sharon and Lisa giving their thoughts on this piece 🙏